Change is Inevitable. Success is Optional
In a highly regulated pharmaceutical landscape, change isn’t just necessary-it’s unavoidable. Whether it's evolving compliance standards, increasing submission volumes, or global market expansions, adapting to change determines success.
At Freyr Digital, we simplify change with a focus on:
Building Trust Through Compliance
Seamless, error-free submissions that meet global regulatory standards.
Streamlining Operations
Automation and intelligent workflows for optimized efficiency and cost savings.
Driving Innovation
Agile, data-driven insights to keep pace with dynamic regulatory landscapes.
Empowering Teams
Equipping your people with user-friendly tools and a culture of digital readiness.
Change isn’t easy, but with the right partner,
it becomes an opportunity
Let’s navigate this transformation together.
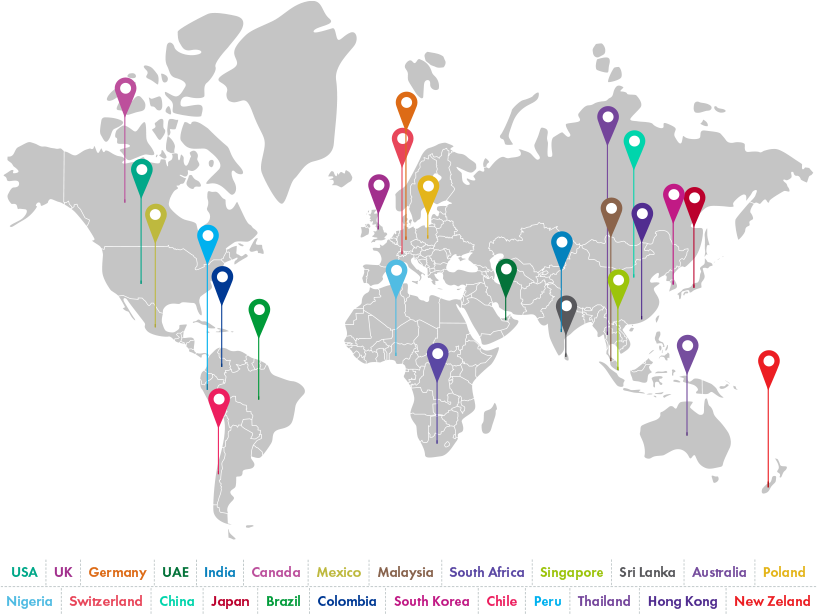
The Scale of Change We Drive
25+
Presence Across 25+ Countries
100,0000+
Successful Submissions
450+
Regulatory Experts
3,350+
Active Users Globally
Every number represents a successful change story-a business that adapted, thrived, and grew.