
In pharma, every lost day in compliance isn’t just a delay - it’s a competitor’s advantage. While legacy systems bury teams in documents, AI-first RIM platforms empower regulatory leaders to accelerate launches, reduce audit risks, and unlock new markets.

This post will walk you through how to architect a future-ready regulatory tech stack — one that scales your business, accelerates filings, fortifies data integrity, and delivers global visibility. Along the way, we’ll spotlight how freya fusion, a unified AI-first RIM platform and its modular solutions enable each layer of your stack, from document management to predictive risk analytics.
Read on — there’s a practical checklist waiting for you.
What’s at stake (right now)
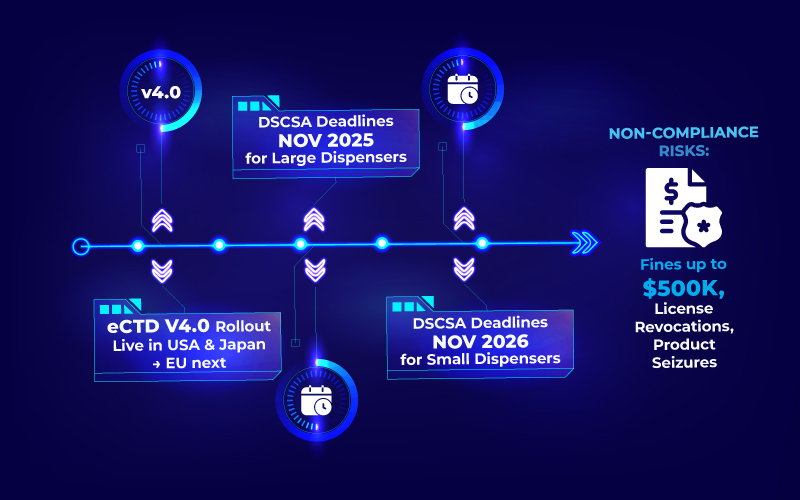
- eCTD v4.0 is live in the US and Japan, with the EU progressing at a slower pace. FDA’s CDER and CBER now accept eCTD v4.0 for new applications, while the EU focuses on updating vocabulary and guidance as part of a phased rollout. Your system should seamlessly manage v3.2.2 today while preparing for a smooth, disruption-free transition to v4.0 tomorrow.
- DSCSA enforcement is phasing in through 2025 – 2026. Large dispensers have flexibility until Nov 27, 2025; small dispensers until Nov 27, 2026. Violation of DSCSA can result in fines upto $250,000 for individuals and up to $500,000 for organizations per violation, along with the risks of imprisonment, license revocations, or product seizures.
- Industry research shows that 96% of clinical and regulatory stakeholders face significant challenges due to siloed systems, with visibility and tracking gaps cited as top productivity blockers
- Regulatory intelligence is shifting from “news” to an actionable strategy. Best-in-class teams use analytics to anticipate changes and model impact — not just read alerts.
The pillars of a modern regulatory stack
A modern regulatory stack should focus on bringing one single source of truth for your data and connecting your teams seamlessly across the globe. From core regulatory standards to AI and emerging tech, here’s the blueprint what you should keep in mind while building your stack and choosing the right partner.
Think of the blueprint not just as a checklist, but as five transformation pillars: Compliance Foundations, Cloud & Security Backbone, AI-Driven Workflows, Interoperability, and Change Management.

Key Regulatory Foundations
Before layering in cutting-edge technologies, it’s critical to anchor your stack on proven regulatory standards:
- FDA 21 CFR Part 11: Ensure all electronic records and signatures meet audit-trail and e-signature requirements.
- ICH Guidelines: From eCTD submissions (ICH M4) to pharmacovigilance (ICH E2B), build templates and workflows that map to global dossier structures.
- DSCSA Traceability: Embed serialization and track-and-trace capabilities across your supply chain nodes to comply with U.S. Drug Supply Chain Security Act mandates.
By codifying these foundations in an integrated RIMS, you eliminate silos, standardize compliance across regions, and expedite audits.
Relevant freya fusion modules
| Regulation | freya fusion Module | Value Outcomes |
|---|---|---|
| eCTD Filings | freya.submit | Automates lifecycle management of global submissions |
| Document Control | freya.docs | One source of truth: 50% faster audits with validated versioning. |
| Labeling Compliance | freya.label | Ensures you can track submissions and Health Authority approvals for each registration in a country |
| Traceability | freya.register | Enables consistency with serialization and track-and-trace data repository |
How it works in reality:
Regulatory teams often struggle with scattered past submissions across systems. By migrating them into a centralized repository, teams can easily reuse data for new applications and quickly reference past records during inspections.
Cloud-Based Compliance Solutions
With the foundations in place, establish the cloud backbone that scales and secures it all. Moving to a cloud-native SaaS model delivers three strategic benefits:
- Scalability: Instantly expand for new products, markets, or M&A — no hardware required.
- Security & Compliance: SOC 2, ISO 27001, and GDPR-ready controls built in.
- Cost Optimization: Predictable subscriptions replace heavy licenses, with seamless updates and validations.
By deploying your RIM on a validated cloud environment, you can rapidly onboard sites, accelerate training, and maintain continuous compliance — even as regulations evolve.
Expert Tip: Select a vendor that provides validation artifacts (IQ/OQ/PQ) and compliance attestations as part of your SaaS subscription to streamline audits and reduce IT overhead.
Digital Transformation Enablers
With your cloud footing set, digitize day-to-day work to remove paper and handoffs.
At the core of a modern tech stack are digital workflows that replace paper and manual handoffs.
- EBRs and QMS bring consistency across manufacturing and QC.
- Unified RIM platforms bring submissions, labeling, change control, and artwork into one system.
With freya.fusion every change is tracked, reviewed, and made audit-ready.ready.
AI & Analytics for Regulatory Compliance
With digital rails laid, layer on intelligence through AI and analytics.
To stay ahead in a data-driven era, embed AI and predictive analytics into compliance workflows:

- Automated document processing with NLP to classify and extract meta data instantly.
- Predictive risk analytics to flag issues before they become findings.
- Real-time dashboards to track submissions, approvals, and regional gaps at a glance.
With freya chatbot — our intelligent regulatory wiz, regulatory teams move from reactive firefighting to proactive, data-driven decision-making.
freya fusion’s AI auto-suggests dossier sections from past submissions, flags cross-regional labeling variations, and forecasts resource needs for HA queries. Unlike bolt-on AI add-ons, freya fusion is AI-first by design — explainable, audit-ready, and regulator-friendly
Insider Insight: Organizations leveraging predictive analytics see up to a 40% reduction in compliance-related delays, as early warnings enable faster corrective actions.
Tech Stack Architecture
Once intelligence is embedded, align the pieces into a clear, layered architecture. A resilient regulatory tech stack is built in five layers:
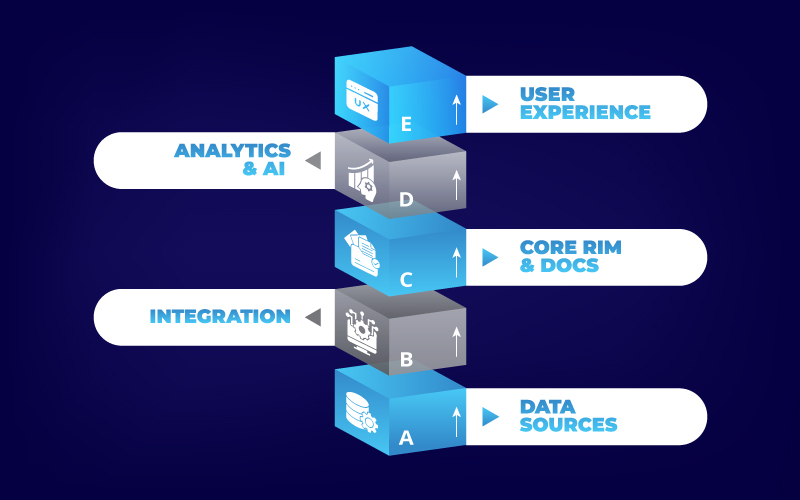
- Data Sources: Clinical databases, publications, intelligence database, and internal repositories, (lab reports, labeling drafts).
- Integration: APIs and ETL pipelines for real-time ingestion, plus blockchain connectors for immutable traceability.
- Core RIM & Document Services: Centralized repository (freya.docs), submission engine (freya.submit), and label management (freya.label).
- Analytics & AI: Visual reports & predictive dashboards (freya) and automated workflows (freya.automate).
- User Experience: Role-based portals for managers, reviewers, and executives, with a fully collaborative platform for on-demand guidance.
This modular architecture ensures you can swap or upgrade components without a full rip-and-replace — future-proofing your investment.
Integration & Interoperability
The architecture is now mapped. Next on, make sure everything talks to everything else — seamlessly.
Fragmented point solutions create data silos, manual handoffs, and version-control nightmares. To achieve seamless interoperability:
- Modular Design: Adopt a plug-and-play approach, enabling you to activate only the modules you need.
- Real-Time Data Exchange: Sync submissions with PV and QMS via REST APIs and web-hooks.
- Scalable Cloud Architecture: Autoscaling micro-services ensure uptime during peak submissions.
By establishing a unified data model across modules, regulatory affairs teams maintain one “source of truth” for compliance data.
Unlike legacy RIMS that depend on bolt-on integrations, freya fusion’s API-first, modular design eliminates silos and ensures seamless interoperability across PV, QMS, and ERP systems.
Validation & Change Management
With systems integrated, lock in quality with validation and ready your teams for change.
A future-ready tech stack isn’t just about software, it also requires:
- Risk-Based Validation (GAMP 5): Focus testing efforts on high-risk workflows and critical quality attributes.
- Personnel Training: Develop role-specific curricula for submission creators, reviewers, and IT teams.
- Process Redesign: Map existing SOPs to new digital workflows, identifying opportunities to retire redundant tasks.
Freyr’s professional services team provides pre-built SOP templates and validation accelerators, reducing deployment timelines by up to 30% while ensuring GxP compliance.
Vendor Selection & Implementation Best Practices
Once governance is in place, choose the right partner and a rollout path that builds momentum.
When choosing a regulatory tech partner, focus on:
- Regulatory Expertise: Platforms built by former regulators and industry pros.
- Validation Support: Full IQ/OQ/PQ packages and SOPs included.
- Change Management: Training and process mapping to drive adoption.
- Roadmap Alignment: Commitment to emerging needs like eTMF and RWE guidelines.
A phased implementation — starting with high-impact modules like freya.submit and freya.docs, then layering in advanced analytics—helps build momentum and demonstrates early ROI.
Future Trends in Regulatory Tech
With the program up and running, keep an eye on what’s next to stay ahead. As regulations converge and data volumes surge, four trends will define the next decade:
- Global Harmonization: ICH E8(R1) and eCTD v4.0 to streamline submissions.
- Real-World Evidence (RWE): Merging post-market insights with pre-market filings.
- Expedited Reviews: AI-optimized dossiers for FDA RTOR and EMA PRIME.
- Quantum-safe Security: Next-gen encryption to safeguard regulatory.
From 2025–2030, regulatory teams will evolve from document-driven compliance to structured content and AI-optimized dossiers. freya fusion is designed to scale seamlessly with this transformation.
By partnering with a next-gen platform like freya fusion, regulatory teams can adapt in real time — activating new modules as standards evolve, and emerging use cases arise.
Vendor Checklist (steal this)
- Unified RIM, not a bundle of point tools
- Validation kits (IQ/OQ/PQ) with each release
- Open APIs & webhooks (prove it in a sandbox)
- eCTD v4.0 readiness + structured content path
- AI you control (explainable, audit-ready)
- Change-management (training, SOP templates, adoption plan)
freya fusion hits these by design, with modular adoption, so you win value early and expand on your terms.
Final Thoughts
Building a future-ready regulatory tech stack is a journey. By grounding in standards, adopting cloud-native tools, and adding AI-driven insights, pharma can turn compliance from a cost center into a competitive advantage.
freya fusion acts as your unified AI-first RIM platform — delivering end-to-end regulatory management, real-time visibility, and predictive analytics. Whether you start with freya.submit for submissions or expand into freya.rtq for risk analysis, the modular nature of the platform lets you scale at your own pace.
Ready to transform compliance into competitive advantage? Start with freya fusion, an AI-first RIM platform — built for speed, scale, and global trust.
For tailored implementation support and validation services, visit our professional services team at Freyr Solutions: https://www.freyrsolutions.com/.
Discover how AI forms the core of a future-ready regulatory tech stack. Download our whitepaper to learn how AI is reshaping Regulatory Affairs for global life sciences.

