
For most Regulatory Affairs teams, registration renewal is not just another administrative milestone. It is the moment when years of quality records, post-market data, and change histories come together under regulatory scrutiny.
Yet renewals are often approached reactively.
Files are pulled together late. PMS data lives in different folders. Certificates are tracked in spreadsheets. And suddenly, what should have been a structured process turns into a race against deadlines.
Medical device renewals do not fail because teams lack effort. They fail because renewal planning starts too close to expiry.
A strong renewal strategy changes this. It treats renewal as a continuous lifecycle activity, not a last-minute submission exercise.
Let’s break down what that really means for RA professionals.
Why Medical Device Renewals Need a Strategy
Most device registrations are valid for 3 to 5 years, depending on the market. Some regions follow annual renewals, while others apply retention models with periodic payments. Regardless of the structure, once a license expires, the device cannot be legally manufactured, imported, or distributed.
Renewal submissions are high-risk because authorities reassess:
- Post-market performance
- Complaint trends and adverse events
- Manufacturing and quality system changes
- Clinical and safety evidence
- Regulatory compliance history
In many regions, including India and the EU, regulators expect consolidated PMS data, CAPA evidence, and updated technical documentation as part of renewal review.
This is why renewal should be treated as an operational discipline that runs throughout the product lifecycle.
Not as a deadline-driven task.
Start With a Regulatory Calendar; Your First Line of Defense
One of the most common renewal failures comes from fragmented tracking. Different markets. Different timelines. Different requirements.
A centralized regulatory calendar should capture:
- Certificate numbers and expiry dates by country
- Renewal submission windows
- Retention fee timelines
- ISO 13485 and CE certificate validity
- Internal milestones (12 months, 6 months, 3 months pre-renewal)
From a practical RA standpoint, planning should begin at least 12 months before expiry.
Many teams now use unified RIMS platforms to maintain a single view of registration status across markets, helping avoid spreadsheet dependency while keeping renewal timelines visible and actionable.
freya fusion is first of its kind to allow organizations to have a single source of truth for all their data pertaining to compliance.
Core Documentation You Will Always Need
Renewal documentation closely mirrors initial registration, but with added focus on continuity and performance.
Most authorities expect:
- Cover letter clearly stating intent to renew
- Declaration of continued compliance (manufacturing, design, ownership)
- Updated Device Master File and Plant Master File (if applicable)
- Valid ISO 13485 certificate
- CE certificates or quality assurance approvals where required
- Manufacturing licenses and GMP evidence
- Declaration of Conformity
- Regulatory correspondence history
Any changes during the validity period must already be reported through proper post-approval pathways. Undisclosed changes are one of the fastest ways to invite regulatory questions.
This is where structured document management matters. RA teams benefit when all submissions, revisions, and approvals live in one controlled environment rather than scattered across drives and inboxes.
Post-Market Surveillance Is the Heart of Renewal
If there is one area for regulators to examine closely during renewal, it is post-market surveillance.
Authorities expect proof that the device has continued to perform safely and effectively in real-world use.
Typically, this includes:
- Sales data by year and device variant
- Complaint registers and adverse event reports
- Root cause analysis for each issue
- CAPA records with closure evidence
- Recall history (global and country-specific)
- Trending analysis over the full validity period
Higher-risk devices may also require:
- PMS reports
- Clinical Evaluation Report updates
- PMCF data
- Periodic Safety Update Reports
Strong renewal outcomes depend on how consistently this data is captured over time, not how well it is assembled at the end.
Many RA teams now integrate PMS tracking directly into their regulatory workflows using unified platforms like freya fusion, allowing complaints, CAPAs, and registrations to stay connected for easier traceability during renewal preparation.
Align Your Quality System with Renewal Readiness
Your QMS is not separate from renewal. It is the foundation of renewal.
Regulators look for evidence across key domains such as:
- Document control and version history
- Change management for manufacturing, labeling, and packaging
- Supplier qualification and audits
- Complaint handling and trending
- CAPA effectiveness
- Internal audits
- Training and competency records
A practical approach is to conduct a pre-renewal gap analysis about six months before submission:
- Verify certificates will remain valid through review
- Confirm PMS data completeness
- Check CAPA closures
- Review change logs
- Ensure training records are current
This proactive audit often prevents last-minute corrective actions.
Plan for Multi-Market Complexity Early
Renewals rarely happen in isolation. Global portfolios involve different agents, distributors, notified bodies, and authorities.
Coordination usually includes:
- Manufacturer providing technical files and PMS data
- Local agents managing submissions and licenses
- Distributors sharing sales and complaint inputs
- Notified bodies supplying audit certificates
Without clear ownership and timelines, delays multiply.
A centralized system that tracks jurisdiction-specific requirements, submission status, and regulatory communications can significantly reduce friction. This is where freya fusion supports RA teams by bringing registrations, documents, and stakeholder collaboration into one unified regulatory workspace.
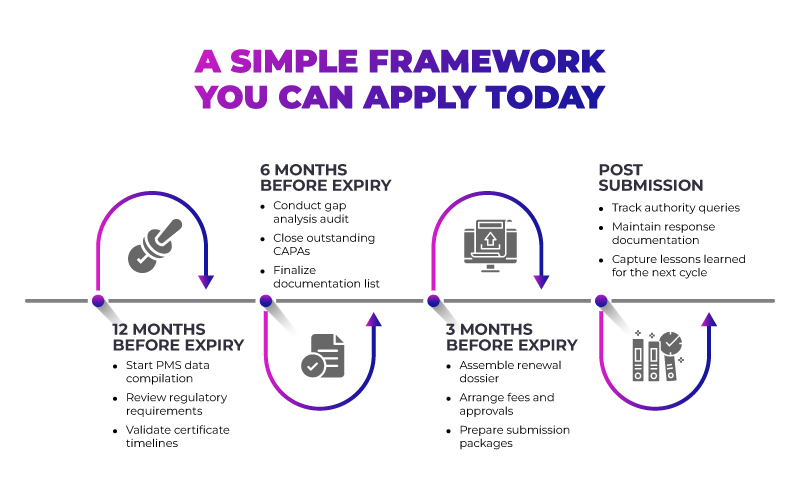
This phased approach turns renewal into a predictable process rather than a recurring crisis.
Renewal Is About Continuity, Not Compliance Alone
Medical device registration renewal is ultimately about protecting uninterrupted market access. When managed strategically, it strengthens quality systems, improves PMS discipline, and builds regulatory confidence.
RA professionals already do the hard work every day. The challenge is giving that work structure, visibility, and continuity.
Here, platforms like freya fusion come in. By unifying registrations, documents, PMS, and collaboration, it helps RA teams move from reactive renewals to proactive regulatory lifecycle management.
Ready to Simplify Your Medical Device Renewals?
If renewals still feel fragmented, manual, or stressful, it might be time to rethink how your regulatory operations are structured.
See how freya fusion, with its AI-First capabilities, supports medical device RA teams with centralized registration tracking, document control, and lifecycle visibility, all designed for regulatory professionals, not IT teams.
Book a demo today and take the first step toward renewal of confidence, continuous compliance, and smoother market continuity.


